this post was submitted on 22 Jul 2024
576 points (96.9% liked)
Science Memes
13145 readers
425 users here now
Welcome to c/science_memes @ Mander.xyz!
A place for majestic STEMLORD peacocking, as well as memes about the realities of working in a lab.

Rules
- Don't throw mud. Behave like an intellectual and remember the human.
- Keep it rooted (on topic).
- No spam.
- Infographics welcome, get schooled.
This is a science community. We use the Dawkins definition of meme.
Research Committee
Other Mander Communities
Science and Research
Biology and Life Sciences
- !abiogenesis@mander.xyz
- !animal-behavior@mander.xyz
- !anthropology@mander.xyz
- !arachnology@mander.xyz
- !balconygardening@slrpnk.net
- !biodiversity@mander.xyz
- !biology@mander.xyz
- !biophysics@mander.xyz
- !botany@mander.xyz
- !ecology@mander.xyz
- !entomology@mander.xyz
- !fermentation@mander.xyz
- !herpetology@mander.xyz
- !houseplants@mander.xyz
- !medicine@mander.xyz
- !microscopy@mander.xyz
- !mycology@mander.xyz
- !nudibranchs@mander.xyz
- !nutrition@mander.xyz
- !palaeoecology@mander.xyz
- !palaeontology@mander.xyz
- !photosynthesis@mander.xyz
- !plantid@mander.xyz
- !plants@mander.xyz
- !reptiles and amphibians@mander.xyz
Physical Sciences
- !astronomy@mander.xyz
- !chemistry@mander.xyz
- !earthscience@mander.xyz
- !geography@mander.xyz
- !geospatial@mander.xyz
- !nuclear@mander.xyz
- !physics@mander.xyz
- !quantum-computing@mander.xyz
- !spectroscopy@mander.xyz
Humanities and Social Sciences
Practical and Applied Sciences
- !exercise-and sports-science@mander.xyz
- !gardening@mander.xyz
- !self sufficiency@mander.xyz
- !soilscience@slrpnk.net
- !terrariums@mander.xyz
- !timelapse@mander.xyz
Memes
Miscellaneous
founded 2 years ago
MODERATORS
you are viewing a single comment's thread
view the rest of the comments
view the rest of the comments
accurate, and for the record, EPA, you can take my DCM wash bottle out of my DEAD DEGREASED HANDS
Like, so what if we store our tBuLi with other low-flash point flammables? And pyrophoric oxidizers? In the same bin? That's stuck in a block of ice in the 30-year-old freezer because it hasn't ever been de-iced?
Haha sounds crazy. And, I wouldn't have to do the shitty quench before disposal. Or work on that project anymore.
Haha, yeah :)
:)
Oh, while you're here, does this still smell like DCM? I can't tell if I rotavapped it all off and the NMR tubes all need aqua regia (sorry my b).
Aqua regia isn't even that scary. Try pipetting pure bromine while it shoots itself out from constantly evaporating
Aqua regia ain't no piranha, and also ain't the most concerning thing in my post lol.
Ah bromine. Super dense, low MW, and low bp, all making dosing accurate amounts a heroic feat. If you store your bromine cold, you can precool the pipette by sucking up and spitting out a few times before transfering, which helps cut down the vapor.
That's just bad management and you shouldn't store tBuLi that long anyway because it'll decompose. You shouldn't put it in freezer either
just put it on high vacuum
What are you working with that requires aqua regia to clean NMR tubes? I've only had to use piranha once in a decade, while cleaning things that acetone, DCM, and basic ethanol won't touch, and this was just after moving to another lab
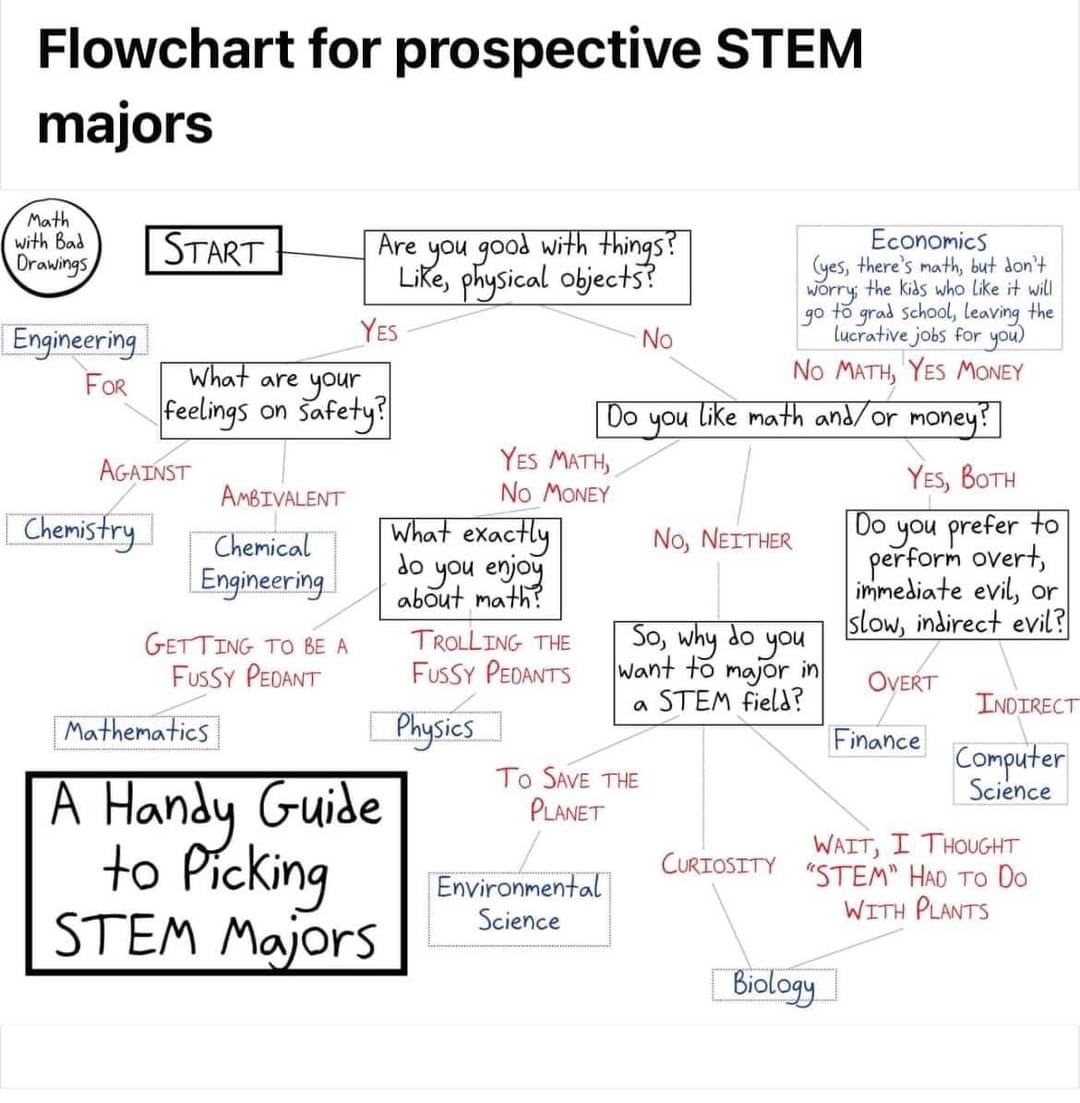
Yes. The whole thing is satirizing the "Safety -> Against" bit. Each piece, though exaggerated for effect, has a basis in something I've seen over the years.
Regarding NMR tubes though, the answer in my old group was precious metal complexes, which have a tendency to mirror out once they've done their bit. Or just existed for too long; a lot of them were touchy. The mirror tends to resist solvents and scrubbing. Nitric acid alone sometimes was enough to remove it depending on the metal, but often not. At some point the cost, effort, and danger are all supposed to outweigh just binning the lot and buying new tubes, but my PI was allergic to buying new things.
Look, I'm all for green chemistry, and I'll switch to using safer, more environmentally friendly reagents and solvents the second they are close to the efficacy of the real deal.
Until then, leave my acetone and heavy-metal catalysts alone!
Acetone is rather green (7 in GSK solvent guide), but I for one haven't used heavy metal catalysts in a year, and more if you don't count palladium
Depends what is meant by green. Acetone is decent for health and safety (flammability notwithstanding) but is produced from petrochemicals and tied to the production of phenol (petroleum -> benzene and propane (or natural gas -> propane), propane -> propylene, benzene + propylene -> cumene, cumene + O2 -> phenol + acetone). Not much chlorophyll involved. Also has somewhere between a moderate to obscene CO2 burden depending on how you draw that box in and around the oil industry, but so do most commodity chemicals.
Maybe not directly, but a lot of commodity chemicals rely on some truly vile metal mixtures for catalysis :)
Almost all of solvents are made from petrochemicals, so it's a distinction without difference. Few exceptions (bioethanol, MeTHF, DMSO maybe) also require obscene amounts of energy to prepare
What makes acetone tick as a solvent is the fact that most of phenol is used up immediately for bisphenol A, and this leaves 1eq of acetone from cumene process unused (or cyclohexanone, and this leaves all acetone unused). In some way, acetone is a waste product and that's why PMMA or isophorone diamine is a thing
Just it's low toxicity, the fact it's non-chlorinated and sane boiling point makes acetone a pretty green solvent by comparison
When I find a solvent on pubchem that has the taste characterized by some mad lad from the 1800s, it makes me want to try it.
You say THF is spicy water? Now I'm curious. We must confirm this claim.
I hear ether smells good. We must confirm this claim.
These fancy new box cutters are safe and cannot cut your hand. We must confirm this claim.