this post was submitted on 30 Jan 2024
143 points (87.8% liked)
Space
8789 readers
19 users here now
Share & discuss informative content on: Astrophysics, Cosmology, Space Exploration, Planetary Science and Astrobiology.
Rules
- Be respectful and inclusive.
- No harassment, hate speech, or trolling.
- Engage in constructive discussions.
- Share relevant content.
- Follow guidelines and moderators' instructions.
- Use appropriate language and tone.
- Report violations.
- Foster a continuous learning environment.
Picture of the Day
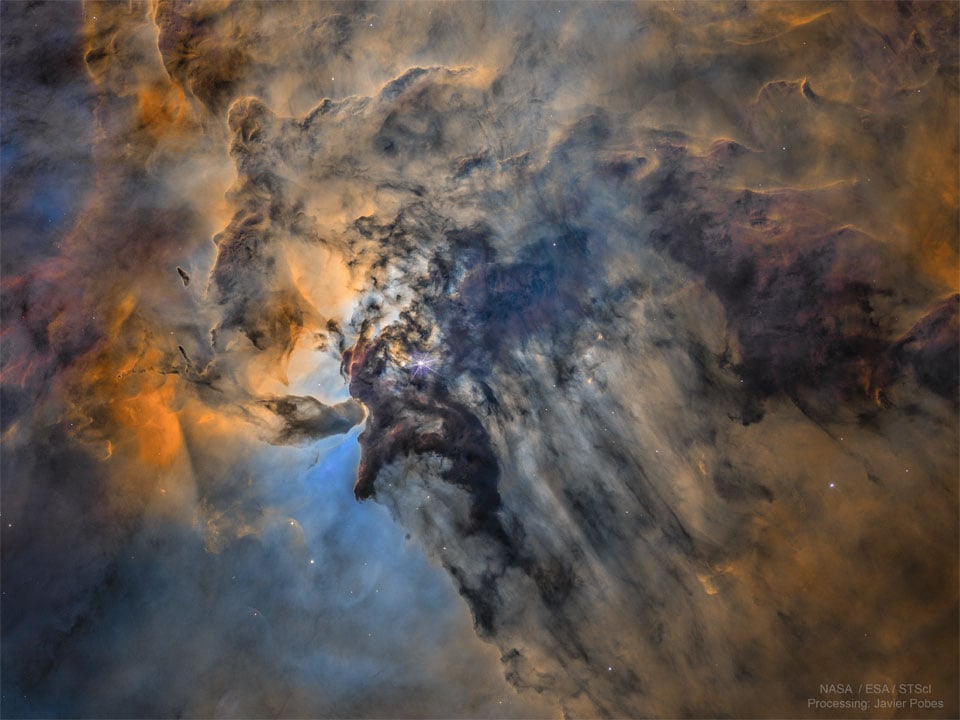 The Busy Center of the Lagoon Nebula
The Busy Center of the Lagoon Nebula
Related Communities
🔭 Science
- !astronomy@mander.xyz
- !curiosityrover@lemmy.world
- !earthscience@mander.xyz
- !esa@feddit.nl
- !nasa@lemmy.world
- !perseverancerover@lemmy.world
- !physics@mander.xyz
- !space@beehaw.org
- !space@lemmy.world
🚀 Engineering
🌌 Art and Photography
Other Cool Links
founded 1 year ago
MODERATORS
you are viewing a single comment's thread
view the rest of the comments
view the rest of the comments

thanks.. and 3: not everything is always serious
No mining of the Moon. Leave it alone. If we are to explore (not exploit) space, do it sustainably.
Getting water in space for making fuel and oxygen, drinking, sanitation and growing plants will be way more sustainable than lifting water off of Earth for use in space.
Water is an absolutely essential resource but it's heavy and it doesn't compress (you can't make it smaller to fit on a rocket). Lifting water mass into orbit is ridiculously expensive in terms of rocket fuel and vehicle use.
The moon is a dead rock. There's no ecology to disturb. It's nearby (comparatively), and a much easier target to land on than an asteroid, but also relatively easy to take off from again after you've landed. We should get water there if we can.
Probably dumb idea, but what about taking hydrogen and oxygen, both of which can be compressed?
Plus, when you make water with them, they go bang, which definitely has some applications
Water is an incredibly space efficient storage for hydrogen and oxygen
The problem is that in gas form hydrogen and oxygen take up more volume than they would as liquid water. To store them compactly you have to cool them to liquid, but that requires a bulky and power-hungry refrigeration system.
Also, hydrogen is a nasty thing to try to store in pure form. You have to deal with embrittlement. Hydrogen has one electron which it easily gives up to form a chemical bond with whatever happens to be around... such as the walls of the storage vessel or the seal around the valve or whatever the valve is made of... and that bond degrades the integrity of the container and eventually it leaks, and then you have a pure hydrogen leak to deal with. Most applications that need hydrogen try to generate it as close to the time of use as possible. Trying to keep pure hydrogen in a tank sucks.
Hydrogen + oxygen is the most energetic chemical reaction you can get, which makes it effective for rocket propulsion. But there are other fuels used such as RP-1 (refined kerosene) because they're less of a PITA to deal with than hydrogen and depending on your rocket design you might get better efficiency if you don't have to carry the extra weight of the hydrogen cooling and storage system.
Mass is mass
People forget that no matter how you stack it. It is only the number of protons and neutrons that matter.
Sustainability loses it's meaning in space, or needs a whole different meaning. There are no ecosystems on other planets or moons which we could tip over with unsustainable practices.
Just throwing this out without addressing the glaring differences looks like uninformed romanticism.